Meso enyne substituted BODIPYs: synthesis, structure and properties†
Abstract
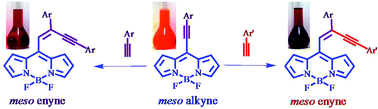
We report the synthesis of meso enyne substituted BODIPYs by the reaction of 8-chloro BODIPY with terminal alkynes under Sonogashira coupling conditions, and by Pd–Cu catalyzed hydroalkynylation reaction of terminal alkynes, across the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– bond of meso alkynylated BODIPYs. The scope of reaction was explored by reacting different meso alkynylated BODIPYs with various terminal alkynes, which results in meso enyne substituted BODIPYs with different substituents. The meso enyne substituted BODIPYs show blue shifted absorption and red shifted emission with large Stokes shift compared to meso alkynylated BODIPYs. The single crystal structures of BODIPYs 2a, 3b, 4a and 2d are reported. Their packing diagram exhibits extensive intermolecular C–H⋯π, C–H⋯F hydrogen bonding and π⋯π stacking interactions, leading to 1D supramolecular frameworks extending into the complex 3D structural frameworks.
C– bond of meso alkynylated BODIPYs. The scope of reaction was explored by reacting different meso alkynylated BODIPYs with various terminal alkynes, which results in meso enyne substituted BODIPYs with different substituents. The meso enyne substituted BODIPYs show blue shifted absorption and red shifted emission with large Stokes shift compared to meso alkynylated BODIPYs. The single crystal structures of BODIPYs 2a, 3b, 4a and 2d are reported. Their packing diagram exhibits extensive intermolecular C–H⋯π, C–H⋯F hydrogen bonding and π⋯π stacking interactions, leading to 1D supramolecular frameworks extending into the complex 3D structural frameworks.

 Please wait while we load your content...
Please wait while we load your content...