Synthesis of a phosphapyracene via metal-mediated cyclization: structural and reactivity effects of acenaphthene precursors†
Abstract
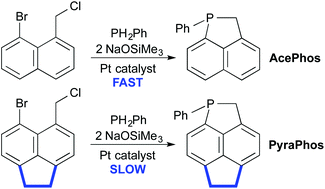
Metal-mediated synthesis of a new heterocycle, 1-phenyl-phosphapyracene (Ph-4, Ph-PyraPhos), by tandem phosphination/cyclization of peri-substituted 5-bromo-6-chloromethylacenaphthene (3) was investigated for comparison to Pt-catalyzed formation of 1-phosphaacenaphthenes (2, AcePhos) from the analogous naphthalene precursor (1). Reaction of PH2Ph with 3, NaOSiMe3 and a Cu catalyst gave Ph-4; a Pt catalyst yielded PHPh(CH2Ar) (Ph-11, Ar = 5-Br-acenaphthyl). Deprotonation of a complex of this secondary phosphine, [Pt((R,R)-Me-DuPhos)(Ph)(PHPh(CH2Ar))][PF6] (17), generated the phosphido intermediate Pt((R,R)-Me-DuPhos)(Ph)(PPhCH2Ar) (Ph-8), which cyclized to give [Pt((R,R)-Me-DuPhos)(Ph)(Ph-PyraPhos)][PF6] (18). Treatment of Ph-8 with silver triflate gave 18 and the cyclometalated phosphine complex [Pt((R,R)-Me-DuPhos)(κ2-(P,C)-5-Ph2PCH2-6-C12H8)][PF6] (21), which might form via Pt(IV) intermediates. The effects of the added “ace” bridge on structure and reactivity are discussed.

 Please wait while we load your content...
Please wait while we load your content...