Syntheses, structure solutions, and catalytic performance of two novel layered silicates†
Abstract
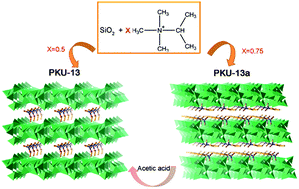
Two novel layered silicates, PKU-13 and PKU-13a, were hydrothermally synthesized by using trimethylpropylammonium hydroxide as the structure directing agent (SDA). Their structures were solved by using powder X-ray diffraction data in combination with electron diffraction technique and NMR spectroscopy. These two silicates are built from the same r52 layer in different stacking modes: the adjacent r52 layers in PKU-13a have a 0.5b + 0.68c shift compared with those in PKU-13. The difference is due to the SDA cations located between the layers. The SDA cations exist as a monolayer in the structure of PKU-13, and link the adjacent layers by Coulomb actions in combination with strong hydrogen bonds. In PKU-13a, the SDA cations present in the bi-layer expend the distance between layers and destroy the inter-layer hydrogen bonds. PKU-13a can transform to PKU-13 after treatment with acetic acid solution. The co-existence of intra-layer hydrogen bonds in PKU-13 interfere in its condensation to an ordered crystalline microporous framework. Both PKU-13 and PKU-13a exhibit good catalytic activities as base catalysts in the Knoevenagel condensation reaction.

 Please wait while we load your content...
Please wait while we load your content...