Ru(EDTA) mediated partial reduction of O2 by H2S†
Abstract
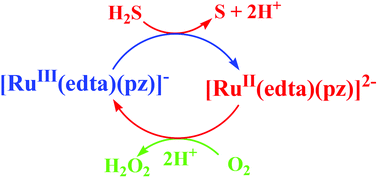
An effective procedure for selective reduction of O2 to H2O2 exploring the use of hydrogen sulfide, an obnoxious industrial pollutant as reductant is reported herein. The reduction of [RuIII(EDTA)pz]− (EDTA4− = ethylenediaminetetraacetate; pz = pyrazine) by hydrogen sulfide resulting in the formation of a red [RuII(EDTA)pz]2− complex (λmax = 462 nm) has been studied spectrophotometrically and kinetically using both rapid scan and stopped-flow techniques. The time course of the reaction was followed as a function of [HS−]i, pH (5.5–8.5), and temperature. Alkali metal ions were found to have a positive influence (K+ > Na+ > Li+) on the reaction rate. Kinetic data and activation parameters are interpreted in terms of a mechanism (admittedly speculative) involving outer-sphere electron transfer between the reaction partners. Reaction of the red [RuII(EDTA)pz]2− complex with molecular oxygen regenerates the [RuIII(EDTA)pz]− species in the reacting system along with the formation of H2O2, a partially reduced product of dioxygen (O2) reduction. A detailed reaction mechanism in agreement with the spectral and kinetic data is presented.

 Please wait while we load your content...
Please wait while we load your content...