Iminoborylene complexes: evaluation of synthetic routes towards BN-allenylidenes and unexpected reactivity towards carbodiimides†‡
Abstract
The synthetic and reaction chemistries of cationic iminoborylene complexes [LnM![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
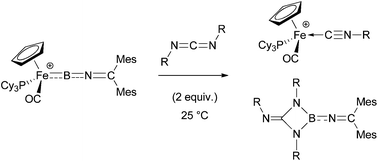
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR2]+, which feature a unique heterocumulene structure, have been systematically investigated. Precursors of the type CpFe(CO)2B(Cl)NCAr2 (Ar = p-Tol/Mes, 5c/d) have been generated by B-centred substitution chemistry using CpFe(CO)2BCl2 and suitable lithiated ketimines – a reaction which is found to be highly sensitive to the steric bulk at both the metal fragment and the ketimino group. Carbonyl/phosphine exchange (using PCy3 or PPh3), followed by halide abstraction allows for the generation of the cationic iminoborylenes [CpFe(PR3)(CO)(BNCAr2)]+[BArX4]− (R = Cy, Ar = p-Tol/Mes, 12c/d; R = Ph, Ar = Mes, 13d; ArX = 3,5-X2C6H3 where X = Cl, CF3) which have been characterized spectroscopically and by X-ray crystallography. The reactivity of these iminoborylene systems towards a range of nucleophiles and unsaturated substrates has been investigated. The latter includes the first examples of M
CR2]+, which feature a unique heterocumulene structure, have been systematically investigated. Precursors of the type CpFe(CO)2B(Cl)NCAr2 (Ar = p-Tol/Mes, 5c/d) have been generated by B-centred substitution chemistry using CpFe(CO)2BCl2 and suitable lithiated ketimines – a reaction which is found to be highly sensitive to the steric bulk at both the metal fragment and the ketimino group. Carbonyl/phosphine exchange (using PCy3 or PPh3), followed by halide abstraction allows for the generation of the cationic iminoborylenes [CpFe(PR3)(CO)(BNCAr2)]+[BArX4]− (R = Cy, Ar = p-Tol/Mes, 12c/d; R = Ph, Ar = Mes, 13d; ArX = 3,5-X2C6H3 where X = Cl, CF3) which have been characterized spectroscopically and by X-ray crystallography. The reactivity of these iminoborylene systems towards a range of nucleophiles and unsaturated substrates has been investigated. The latter includes the first examples of M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B metathesis reactivity with a carbodiimide, and results in Fe
B metathesis reactivity with a carbodiimide, and results in Fe![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B cleavage and formation of the isonitrile complexes [CpFe(PCy3)(CO)(CNR)]+[BArCl4]− (R = iPr/Cy, 16/17).
B cleavage and formation of the isonitrile complexes [CpFe(PCy3)(CO)(CNR)]+[BArCl4]− (R = iPr/Cy, 16/17).
- This article is part of the themed collection: In memory of Professor Kenneth Wade

 Please wait while we load your content...
Please wait while we load your content...