Iron catalysed Negishi cross-coupling using simple ethyl-monophosphines†
Abstract
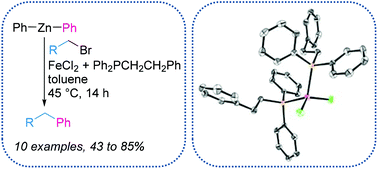
Monophosphines prepared by iron catalysed hydrophosphination have been used as pro-ligands in iron catalysed Negishi cross-coupling of alkyl bromides and diphenyl zinc reagents. The cross-coupling has been investigated with monophosphines with varying electronic properties and we find the simplest, unsubstituted phosphine to offer the optimum reaction conditions (both in terms of yield of diarylmethane product and cost-effectiveness of the phosphine). In situ catalyst generation from monophosphine and FeCl2 was used in catalysis; however, preparation of a discrete homonuclear iron complex was also achieved and this four-coordinate iron-phosphine complex was isolated and used in catalysis.
- This article is part of the themed collection: Earth Abundant Element Compounds in Homogeneous Catalysis

 Please wait while we load your content...
Please wait while we load your content...