Oxidation of germa- and stanna-closo-dodecaborate†
Abstract
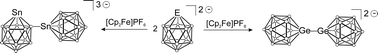
The oxidation of closo-heteroborates [GeB11H11]2− and [SnB11H11]2− is presented. Upon oxidation germa-closo-dodecaborate yields a symmetrical dimer exhibiting a Ge–Ge bond between two clusters. This dimer shows sulphur insertion into the Ge–Ge bond at room temperature. In contrast, oxidation of the homologous tin cluster results in an unsymmetrical dimer bearing an Sn–B bond between two clusters. The Sn–B dimer is also the product of the hydride abstraction reaction. In the presence of the donor ligand 2,2′-bipyridine, the oxidation of closo-cluster [SnB11H11]2− leads to the Sn(IV)-half sandwich coordination compound [bipy-SnB11H11] which dissolves in DMSO to give the Sn(IV)-adduct [bipy(DMSO)-SnB11H11].
- This article is part of the themed collection: In memory of Professor Kenneth Wade

 Please wait while we load your content...
Please wait while we load your content...