Isoquinoline-based Werner clathrates with xylene isomers: aromatic interactions vs. molecular flexibility†
Abstract
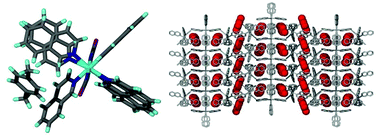
The crystal structures of the Werner clathrates Ni(NCS)2(isoquinoline)4 (H) with para-xylene (px), meta-xylene (mx) and ortho-xylene (ox) have been elucidated. The kinetics of thermal decomposition of the three inclusion compounds were performed using the isothermal technique of Flynn and Wall. Selectivity of H for the xylene isomers was determined for both the liquid and vapour phase binary mixtures of the xylenes. The chosen ligand has a larger aromatic system to improve the possible π interactions between H and the selected guests. The planarity of the isoquinoline ligand causes H rigidity and its selectivity was compared to a related Werner complex containing the more flexible 4-phenylpyridine.

 Please wait while we load your content...
Please wait while we load your content...