Lewis acidity enhancement of triarylborane by appended phosphine oxide groups†
Abstract
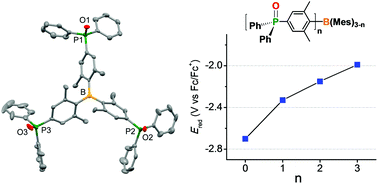
A series of mono-, di-, and tri-phosphine oxide substituted triarylboranes, Mes2BAr (1), MesBAr2 (2), and BAr3 (3) (Ar = 4-(Ph2PO)-2,6-Me2-C6H2) were prepared to investigate the effect of a phosphine oxide group (Ph2PO) on Lewis acidity enhancement of triarylboranes. The X-ray crystal structure of 3 revealed peripheral decoration of Ph2PO groups with a C3-axis perpendicular to the trigonal boron center. UV/Vis absorption and PL spectra indicated a significant contribution of π(Mes or phenylene) → pπ(B) charge transfer in the lower-energy electronic transition. The reduction potential measured by cyclic voltammetry showed apparent LUMO stabilization by introduction of phosphine oxide groups, the extent of which gradually increased with the increasing number of phosphine oxide groups. Lewis acidity enhancement was also supported by the gradual increase in fluoride ion affinity in the order 3 > 2 > 1. Theoretical calculations suggest that introduction of a Ph2PO group into a triarylborane significantly enhances the Lewis acidity of the boron center via an inductive electron-withdrawing effect and this effect is additive for multiple phosphine oxide groups.

 Please wait while we load your content...
Please wait while we load your content...