An anionic phosphenium complex as an ambident nucleophile†
Abstract
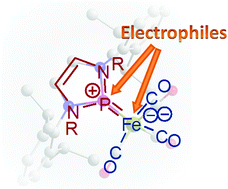
A unique anionic phosphenium complex was prepared from reaction of an N-heterocyclic chlorophosphine with Collman's reagent or K[HFe(CO)4]/NaH and characterized by spectral and XRD data. The complex behaves as an ambident nucleophile. Reactions with acetic acid, ClSnPh3, and a further equivalent of an N-heterocyclic chlorophosphine proceed via electrophilic functionalization at the metal site to yield appropriate mono- or bis-phosphenium complexes. Reaction with MeI at −70 °C produces a P-alkylation product as the first spectroscopically detectable intermediate, which decays at a higher temperature to give a mixture of free P-methylated N-heterocyclic phosphine and its Fe(CO)4 complex. The different reaction products were characterized by spectral and XRD data. Computational studies indicate that the NHP units in all complexes display π-acceptor behaviour but show no disposition to adopt phosphide-like character or formally oxidize the metal centre.

 Please wait while we load your content...
Please wait while we load your content...