2-Adamantylidene and its heavier analogues: hyperconjugation versus lone pair stability and electrophilicity versus nucleophilicity†
Abstract
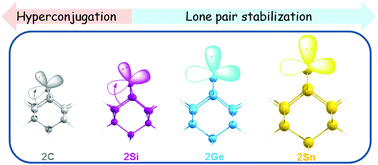
The structure, bonding and reactivity of 2-adamantylidene and its heavier group-14 analogues, 2X (X10H14, X = C–Sn), have been studied at the M06/def2-TZVPP//BP86/def2-TZVPP level of theory. 2-Adamantylidene and its heavier analogues, 2X, have a singlet ground state, where the carbene and silylene bridges in 2C and 2Si are bent similarly to the foiled-type carbenes. The bending of the carbene and silylene bridges in 2C and 2Si as well as their stability are mainly attributed to the Cieplak-type hyperconjugative interaction between a pair of vicinal σ-bonds with the empty p-orbital on the carbon and silicon atoms. However, the inertness of the lone pair of the silicon atom also contributes to the stability of 2Si. The extent of hyperconjugative interactions is significantly less in 2Ge and 2Sn and the inertness of the lone pair is the major factor for their stability. The high proton affinity (PA) and hydride affinity (HA) of 2-adamantylidene and its heavier analogues suggest their ambiphilic nature. The high PA and HA of 2X can be attributed to the stabilization of the protonated adduct, 4X, and the hydride adduct, 5X, by the Cieplak-type and Felkin–Anh-type hyperconjugations, respectively. However, the nucleophilicity of 2X decreases and electrophilicity increases when X changes from C to Sn.

 Please wait while we load your content...
Please wait while we load your content...