Nucleophilic substitution in ionizable Fischer thiocarbene complexes: steric effect of the alkyl substituent on the heteroatom†
Abstract
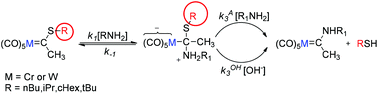
A detailed kinetic study has been carried out for the aminolysis of ionizable Fischer thiocarbene complexes (CO)5M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(SR)CH3 (M = Cr, W; R = iPr, nBu, cHex, tBu) with five primary amines and one secondary amine in aqueous acetonitrile solutions (50% MeCN–50% water (v/v)). The observed rate constants for the reaction with primary amines showed a first-order dependence on the amine concentration, while with morpholine, the rate constant has second-order dependence. The general base catalysis process was confirmed by the variation of the rate constants with the concentration of an external catalyst and the pH. The results agree with a stepwise mechanism where the nucleophilic addition to the carbene carbon to produce a tetrahedral intermediate (T±) is the first step, followed by a rapid deprotonation of T± to form the anion T− which leads to the products by general-acid catalysed leaving group (–SR) expulsion. In general, it was found that the chromium complexes are less reactive than the tungsten analogues. The obtained Brønsted parameters for the nucleophilic addition (βnuc) indicate that C–N bond formation has made little progress at the transition state. By using Charton's correlation, the role that the steric factor plays throughout the mechanism has been unraveled. The nucleophilic addition to the thiocarbenes is less sensitive to steric effects than the alkoxycarbenes regardless of the nature of the metal centre. Conversely, the steric effects on the general-base catalysis can be strong depending on the volume of the catalyst and the metal centre. On the basis of the structure–reactivity coefficients β and ψ and comparison with alkoxycarbene complexes, esters and thiolesters, insights into the main factors ruling the reactivity in terms of transition state imbalances are discussed.
C(SR)CH3 (M = Cr, W; R = iPr, nBu, cHex, tBu) with five primary amines and one secondary amine in aqueous acetonitrile solutions (50% MeCN–50% water (v/v)). The observed rate constants for the reaction with primary amines showed a first-order dependence on the amine concentration, while with morpholine, the rate constant has second-order dependence. The general base catalysis process was confirmed by the variation of the rate constants with the concentration of an external catalyst and the pH. The results agree with a stepwise mechanism where the nucleophilic addition to the carbene carbon to produce a tetrahedral intermediate (T±) is the first step, followed by a rapid deprotonation of T± to form the anion T− which leads to the products by general-acid catalysed leaving group (–SR) expulsion. In general, it was found that the chromium complexes are less reactive than the tungsten analogues. The obtained Brønsted parameters for the nucleophilic addition (βnuc) indicate that C–N bond formation has made little progress at the transition state. By using Charton's correlation, the role that the steric factor plays throughout the mechanism has been unraveled. The nucleophilic addition to the thiocarbenes is less sensitive to steric effects than the alkoxycarbenes regardless of the nature of the metal centre. Conversely, the steric effects on the general-base catalysis can be strong depending on the volume of the catalyst and the metal centre. On the basis of the structure–reactivity coefficients β and ψ and comparison with alkoxycarbene complexes, esters and thiolesters, insights into the main factors ruling the reactivity in terms of transition state imbalances are discussed.

 Please wait while we load your content...
Please wait while we load your content...