Uninterrupted galvanic reaction for scalable and rapid synthesis of metallic and bimetallic sponges/dendrites as efficient catalysts for 4-nitrophenol reduction†
Abstract
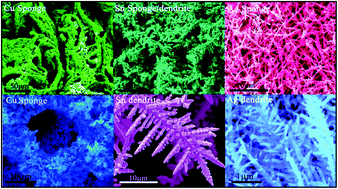
Here, we demonstrate an uninterrupted galvanic replacement reaction (GRR) for the synthesis of metallic (Ag, Cu and Sn) and bimetallic (Cu–M, M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ag, Au, Pt and Pd) sponges/dendrites by sacrificing the low reduction potential metals (Mg in our case) in acidic medium. The acidic medium prevents the oxide formation on Mg surface and facilitates the uninterrupted reaction. The morphology of dendritic/spongy structures is controlled by the volume of acid used for this reaction. The growth mechanism of the spongy/dendritic microstructures is explained by diffusion-limited aggregate model (DLA), which is also largely affected by the volume of acid. The significance of this method is that the yield can be easily predicted, which is a major challenge for the commercialization of the products. Furthermore, the synthesis is complete in 1–2 minutes at room temperature. We show that the sponges/dendrites efficiently act as catalysts to reduce 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) using NaBH4–a widely studied conversion process.
Ag, Au, Pt and Pd) sponges/dendrites by sacrificing the low reduction potential metals (Mg in our case) in acidic medium. The acidic medium prevents the oxide formation on Mg surface and facilitates the uninterrupted reaction. The morphology of dendritic/spongy structures is controlled by the volume of acid used for this reaction. The growth mechanism of the spongy/dendritic microstructures is explained by diffusion-limited aggregate model (DLA), which is also largely affected by the volume of acid. The significance of this method is that the yield can be easily predicted, which is a major challenge for the commercialization of the products. Furthermore, the synthesis is complete in 1–2 minutes at room temperature. We show that the sponges/dendrites efficiently act as catalysts to reduce 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) using NaBH4–a widely studied conversion process.

 Please wait while we load your content...
Please wait while we load your content...