Synthesis, structure, magnetic properties and EPR spectroscopy of a copper(ii) coordination polymer with a ditopic hydrazone ligand and acetate bridges†
Abstract
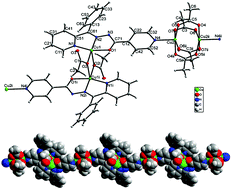
A new one dimensional coordination polymer of copper(II), [Cu4(L)2(μ2-1,1-OAc)2(μ2-1,3-OAc)4]n (1), has been synthesized and characterized by spectroscopic methods and single crystal X-ray analysis [HL = (E)-N′-(phenyl(pyridin-2-yl)methylene)isonicotinhydrazide, OAc = acetate anion]. The coordination polymer contains two kinds of Cu(II) dimers which are connected by two types of acetate (μ2-1,1- and μ2-1,3-) bridging groups. The ditopic isonicotinhydrazone ligand coordinates to the Cu1 center through the N2O-donor set and connects to the Cu2 center by a pyridine group of the isonicotine part. The EPR and magnetic susceptibility measurements confirm the existence of two kinds of Cu(II) dimers. The intradimer isotropic exchange was estimated to be +0.80(1) cm−1 for the ferromagnetic Cu1⋯Cu1 dimeric unit and −315 (1) cm−1 for the antiferromagnetic Cu2⋯Cu2 dimeric unit.

 Please wait while we load your content...
Please wait while we load your content...