Iridium(i) PNP complexes in the sp3 C–H bond activation of methyl propanoate and related esters†
Abstract
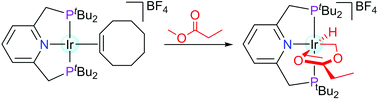
The utilisation of the PNP iridium pincer complex [Ir(PNP)(COE)][BF4] [PNP = 2,6-bis{(di-tert-butylphosphino)methyl}pyridine; COE = cyclooctene] in the sp3 C–H activation of methyl propanoate and other related esters was explored. In particular, this study provides further insight into the factors that govern the regioselectivity of such reactions. These included factors such as the steric demands of the substrate, the formation of favourable ring systems as well as the electronic effects that may influence the pKa values of protons. In particular, the effects of water on the outcome of these reactions were of great interest, since earlier literature reports have shown the presence of water to promote selective C–H activation in the α-position of ketones.
- This article is part of the themed collection: In memory of Professor Kenneth Wade

 Please wait while we load your content...
Please wait while we load your content...