Acidity enhancement of unsaturated bases of group 15 by association with borane and beryllium dihydride. Unexpected boron and beryllium Brønsted acids†
Abstract
The intrinsic acidity of CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHXH2, HC
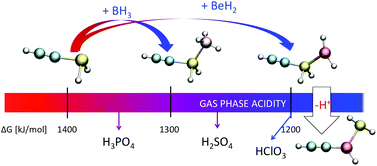
CHXH2, HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CXH2 (X = N, P, As, Sb) derivatives and of their complexes with BeH2 and BH3 has been investigated by means of high-level density functional theory and molecular orbital ab initio calculations, using as a reference the ethyl saturated analogues. The acidity of the free systems steadily increases down the group for the three series of derivatives, ethyl, vinyl and ethynyl. The association with both beryllium dihydride and borane leads to a very significant acidity enhancement, being larger for BeH2 than for BH3 complexes. This acidity enhancement, for the unsaturated compounds, is accompanied by a change in the acidity trends down the group, which do not steadily decrease but present a minimum value for both the vinyl- and the ethynyl-phosphine. When the molecule acting as the Lewis acid is beryllium dihydride, the π-type complexes in which the BeH2 molecules interact with the double or triple bond are found, in some cases, to be more stable, in terms of free energies, than the conventional complexes in which the attachment takes place at the heteroatom, X. The most important finding, however, is that P, As, and Sb ethynyl complexes with BeH2 do not behave as P, As, or Sb Brønsted acids, but unexpectedly as Be acids.
CXH2 (X = N, P, As, Sb) derivatives and of their complexes with BeH2 and BH3 has been investigated by means of high-level density functional theory and molecular orbital ab initio calculations, using as a reference the ethyl saturated analogues. The acidity of the free systems steadily increases down the group for the three series of derivatives, ethyl, vinyl and ethynyl. The association with both beryllium dihydride and borane leads to a very significant acidity enhancement, being larger for BeH2 than for BH3 complexes. This acidity enhancement, for the unsaturated compounds, is accompanied by a change in the acidity trends down the group, which do not steadily decrease but present a minimum value for both the vinyl- and the ethynyl-phosphine. When the molecule acting as the Lewis acid is beryllium dihydride, the π-type complexes in which the BeH2 molecules interact with the double or triple bond are found, in some cases, to be more stable, in terms of free energies, than the conventional complexes in which the attachment takes place at the heteroatom, X. The most important finding, however, is that P, As, and Sb ethynyl complexes with BeH2 do not behave as P, As, or Sb Brønsted acids, but unexpectedly as Be acids.

 Please wait while we load your content...
Please wait while we load your content...