Efficient synthesis of a statin precursor in high space-time yield by a new aldehyde-tolerant aldolase identified from Lactobacillus brevis†
Abstract
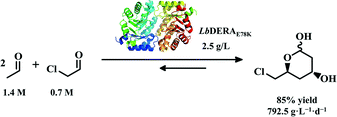
A novel 2-deoxyribose-5-phosphate aldolase (LbDERA) was identified from Lactobacillus brevis, with high activity, excellent thermostability and high tolerance against aldehyde substrates. The half-lives of LbDERA incubated in 300 mM acetaldehyde and chloroacetaldehyde were 37.3 and 198 min, respectively, which are 2- and 7-fold higher than those of EcDERA from Escherichia coli. The crystal structure of LbDERA determined at 1.95 Å resolution revealed a stable quaternary structure which might account for its excellent aldehyde tolerance. A single mutation, E78K, was introduced to LbDERA through a consensus sequence approach, resulting in significant improvements of both thermostability and aldehyde tolerance. According to the crystal structure of LbDERAE78K, two additional hydrogen bonds and one salt bridge were introduced compared with wild-type LbDERA. As a result of its high substrate tolerance, LbDERAE78K could efficiently catalyze a sequential aldol condensation with 0.7 M chloroacetaldehyde and 1.4 M acetaldehyde, affording a key chiral precursor of statins, (3R,5S)-6-chloro-2,4,6-trideoxyhexapyranoside, with an unprecedented space-time yield of 792.5 g L−1 d−1 and only 2.5 g L−1 of catalyst loading.

 Please wait while we load your content...
Please wait while we load your content...