Ozone catalytic oxidation for ammonia removal from simulated air at room temperature†
Abstract
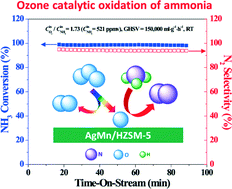
Ozone catalytic oxidation (OZCO) for removing ammonia from simulated air over the AgMn/HZSM-5 (AgMn/HZ) catalyst with high ammonia conversion and high N2 selectivity at room temperature is reported for the first time. HZ, Ag/HZ, Mn/HZ and AgMn/HZ catalysts were compared in the OZCO reactions of gaseous and adsorbed NH3. In OZCO of gaseous NH3, N2 was the major product and N2O was the minor product. NH3 conversion dropped quickly with time-on-stream (TOS) over HZ and Ag/HZ catalysts while it remained almost constant at a high level over Mn/HZ and AgMn/HZ catalysts during the entire test. N2 selectivity of the AgMn/HZ catalyst was higher than that of the Mn/HZ catalyst. When the initial concentration of NH3 was 521 ppmv and the ratio of initial concentration of O3 to NH3 was 1.73, 99% NH3 conversion with 94% N2 selectivity was obtained over the AgMn/HZ catalyst at room temperature and 150 000 ml g−1 h−1 gas hourly space velocity (GHSV). Finally, the pathways for OZCO of NH3 were proposed for the four catalysts based on the comparative investigation of the gaseous products and surface species during OZCO of adsorbed and gaseous NH3.

 Please wait while we load your content...
Please wait while we load your content...