Synergistic catalysis-induced ring-opening reactions of 2-substituted 3,4-dihydropyrans with α-oxoketene dithioacetals†
Abstract
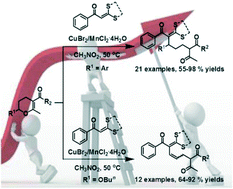
According to the conventional catalytic activation mode, the difficulty of using a less reactive substrate can be alleviated by means of employing a strong catalyst. Because of the susceptibility of the reaction product to strong acid, ring-opening Friedel–Crafts reactions of 2-substituted 3,4-dihydropyrans with a less reactive nucleophile, α-oxoketene dithioacetal, could not be performed by using strong acid as a catalyst. In order to find a suitable catalyst system for this reaction, a co-catalyst, CuBr2, that can increase the reactivity of α-oxoketene dithioacetal was used in conjunction with a moderate Lewis acid, MnCl2·4H2O. The mechanism of synergistic catalysis was also studied with the aid of spectroscopic investigation. It was found for the first time that CuBr2-induced disintegration of a super-conjugation system exists in α-oxoketene dithioacetal and is responsible for the increase of its reactivity. An intramolecular Michael addition of the ring-opening product was also developed, which provided a densely substituted cyclohexane derivative in good yield. Finally, a hitherto unreported S,N-doped eleven-membered heterocycle was synthesized on the basis of the developed reactions.

 Please wait while we load your content...
Please wait while we load your content...