Reversibility of asymmetric catalyzed C–C bond formation by benzoylformate decarboxylase†
Abstract
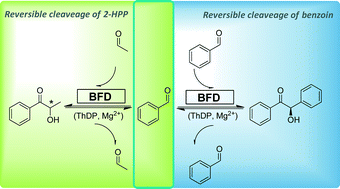
Benzoylformate decarboxylase (BFD) from Pseudomonas putida catalyzed the formation of 2-hydroxy-1-phenylpropanone (2-HPP), a 2-hydroxy ketone, from the kinetic resolution of rac-benzoin in the presence of acetaldehyde. The formation rate of 2-HPP via kinetic resolution of benzoin was 700-fold lower compared to the formation via direct carboligation of benzaldehyde and acetaldehyde. Further investigations revealed that BFD not only accepts (R)-benzoin but also 2-HPP as the substrate. A typical Michaelis–Menten type kinetics was observed starting from enantiopure (S)- or (R)-2-HPP. The formation of racemic 2-HPP while using benzoin as the donor in the presence of acetaldehyde and the racemization of (R/S)-2-HPP were detected. The equilibrium constant determined, showed favoured conditions towards the product side i.e. (R)-benzoin and 2-HPP. In the end, an extended reaction mechanism was proposed by supplementing the already known mechanism with the C–C bond cleavage activity of BFD towards 2-hydroxy ketones.

 Please wait while we load your content...
Please wait while we load your content...