On the atomic structure of cocaine in solution†
Abstract
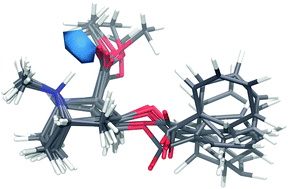
Cocaine is an amphiphilic drug which has the ability to cross the blood–brain barrier (BBB). Here, a combination of neutron diffraction and computation has been used to investigate the atomic scale structure of cocaine in aqueous solutions. Both the observed conformation and hydration of cocaine appear to contribute to its ability to cross hydrophobic layers afforded by the BBB, as the average conformation yields a structure which might allow cocaine to shield its hydrophilic regions from a lipophilic environment. Specifically, the carbonyl oxygens and amine group on cocaine, on average, form ∼5 bonds with the water molecules in the surrounding solvent, and the top 30% of water molecules within 4 Å of cocaine are localized in the cavity formed by an internal hydrogen bond within the cocaine molecule. This water mediated internal hydrogen bonding suggests a mechanism of interaction between cocaine and the BBB that negates the need for deprotonation prior to interaction with the lipophilic portions of this barrier. This finding also has important implications for understanding how neurologically active molecules are able to interact with both the blood stream and BBB and emphasizes the use of structural measurements in solution in order to understand important biological function.


 Please wait while we load your content...
Please wait while we load your content...