Energetic and topological determinants of a phosphorylation-induced disorder-to-order protein conformational switch†
Abstract
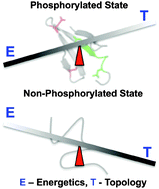
We show that the phosphorylation of 4E-BP2 acts as a triggering event to shape its folding-function landscape that is delicately balanced between conflicting favorable energetics and intrinsically unfavorable topological connectivity. We further provide first evidence that the fitness landscapes of proteins at the threshold of disorder can differ considerably from ordered domains.

 Please wait while we load your content...
Please wait while we load your content...