The hydrogen bond strength of the phenol–phenolate anionic complex: a computational and photoelectron spectroscopic study
Abstract
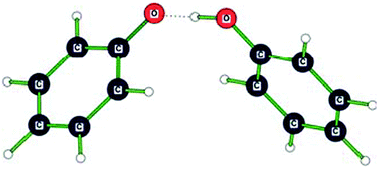
The phenol–phenolate anionic complex was studied in vacuo by negative ion photoelectron spectroscopy using 193 nm photons and by density functional theory (DFT) computations at the ωB97XD/6-311+G(2d,p) level. We characterize the phenol–phenolate anionic complex as a proton-coupled phenolate pair, i.e., as a low-barrier hydrogen bond system. Since the phenol–phenolate anionic complex was studied in the gas phase, its measured hydrogen bond strength is its maximal ionic hydrogen bond strength. The D(PhO−⋯HOPh) interaction energy (26–30 kcal mol−1), i.e., the hydrogen bond strength in the PhO−⋯HOPh complex, is quite substantial. Block-localized wavefunction (BLW) computations reveal that hydrogen bonded phenol rings exhibit increased ring π-electron delocalization energies compared to the free phenol monomer. This additional stabilization may explain the stronger than expected proton donating ability of phenol.

 Please wait while we load your content...
Please wait while we load your content...