Nickel-supported carbon nitride photocatalyst combined with organic dye for visible-light-driven hydrogen evolution from water†
Abstract
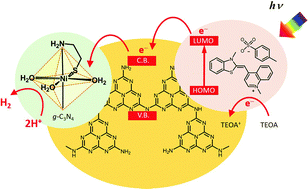
A noble-metal-free photocatalytic H2 production system consisting of a Ni-based catalyst, visible-light-responsive organic dye, and graphitic carbon nitride (g-C3N4) as a support has been developed. Characterization by means of XAFS revealed that the deposition of a trinuclear Ni precursor complex, Ni(NiL2)2Cl2 (L = β-mercaptoethylamine), on the g-C3N4 affords a monomeric Ni(II) species involving β-mercaptoethylamine and aqua ligands in an octahedral coordination geometry. Such a Ni species acts as a hydrogen production site from an aqueous solution without an electron relay reagent by combining with thiazole orange (TO) as a photosensitizer. The emission of the attached TO at around 550 nm decreases with increasing loading amount of Ni catalyst, suggesting electron transfer from TO to the Ni catalyst via the g-C3N4 support. Leaching and agglomeration of the active Ni catalyst and TO are not observed during the photocatalytic reaction. Moreover, the use of highly porous carbon nitride (nanoporous carbon nitride; nanoC3N4) is proven to significantly enhance the photocatalytic activity because of the high surface area due to the unique porous structure as well as high absorption and emission properties of TO associated with nanoC3N4.

 Please wait while we load your content...
Please wait while we load your content...