Physisorption and desorption of H2, HD and D2 on amorphous solid water ice. Effect on mixing isotopologue on statistical population of adsorption sites†
Abstract
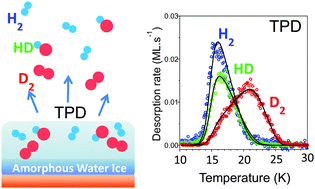
We study the adsorption and desorption of three isotopologues of molecular hydrogen mixed on 10 ML of porous amorphous water ice (ASW) deposited at 10 K. Thermally programmed desorption (TPD) of H2, D2 and HD adsorbed at 10 K have been performed with different mixings. Various coverages of H2, HD and D2 have been explored and a model taking into account all species adsorbed on the surface is presented in detail. The model we propose allows to extract the parameters required to fully reproduce the desorption of H2, HD and D2 for various coverages and mixtures in the sub-monolayer regime. The model is based on a statistical description of the process in a grand-canonical ensemble where adsorbed molecules are described following a Fermi–Dirac distribution.

 Please wait while we load your content...
Please wait while we load your content...