Lysozyme adsorption at a silica surface using simulation and experiment: effects of pH on protein layer structure†
Abstract
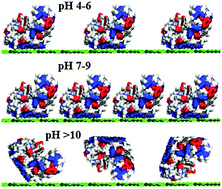
Hen Egg White Lysozyme (HEWL) is a widely used exemplar to study protein adsorption on surfaces and interfaces. Here we use fully atomistic Molecular Dynamics (MD) simulations, Multi-Parametric Surface Plasmon Resonance (MP-SPR), contact angle and zeta potential measurements to study HEWL adsorption at a silica surface. The simulations provide a detailed description of the adsorption mechanism and indicate that at pH7 the main adsorption driving force is electrostatics, supplemented by weaker hydrophobic forces. Moreover, they reveal the preferred orientation of the adsorbed protein and show that its structure is only slightly altered at the interface with the surface. This provides the basis for interpreting the experimental results, which indicate the surface adsorbs a close-packed monolayer at about pH10 where the surface has a large negative zeta potential and the HEWL is positively charged. At higher pH, the adsorption amount of the protein layer is greatly reduced due to the loss of charge on the protein. At lower pH, the smaller zeta potential of the surface leads to lower HEWL adsorption. These interpretations are complemented by the contact angle measurements that show how the hydrophobicity of the surface is greatest when the surface coverage is highest. The simulations provide details of the hydrophobic residues exposed to solution by the adsorbed HEWL, completing the picture of the protein layer structure.

 Please wait while we load your content...
Please wait while we load your content...