The oxidation of copper catalysts during ethylene epoxidation†
Abstract
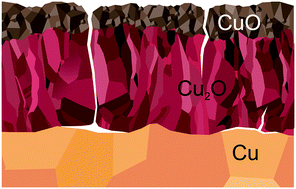
The oxidation of copper catalysts during ethylene epoxidation was characterized using in situ photoemission spectroscopy and electron microscopy. Gas chromatography, proton-transfer reaction mass spectrometry and electron-ionization mass spectrometry were used to characterize the catalytic properties of the oxidized copper. We find that copper corrodes during epoxidation in a 1 : 1 mixture of oxygen and ethylene. The catalyst corrosion passes through several stages, beginning with the formation of an O-terminated surface, followed by the formation of Cu2O scale and eventually a CuO scale. The oxidized catalyst exhibits measurable activity for ethylene epoxidation, but with a low selectivity of <3%. Tests on pure Cu2O and CuO powders confirm that the oxides intrinsically exhibit partial-oxidation activity. Cu2O was found to form acetaldehyde and ethylene epoxide in roughly equal amounts (1.0% and 1.2% respectively), while CuO was found to form much less ethyl aldehyde than ethylene epoxide (0.1% and 1.0%, respectively). Metallic copper catalysts were examined in extreme dilute-O2 epoxidation conditions to try and keep the catalyst from oxidizing during the reaction. It was found that in feed of 1 part O2 to 2500 parts C2H4 (PO2 = 1.2 × 10−4 mbar) the copper surface becomes O-terminated. The O-terminated surface was found to exhibit partial-oxidation selectivity similar to that of Cu2O. With increasing O2 concentration (>8/2500) Cu2O forms and eventually covers the surface.

 Please wait while we load your content...
Please wait while we load your content...