Association of small aromatic molecules with PAMAM dendrimers
Abstract
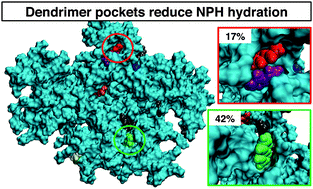
Many proposed applications using dendrimers, such as drug delivery and environmental remediation, involve dendrimer interactions with small molecules. Understanding the details of these interactions is important for designing dendrimers with tunable association with guest molecules. In this work, we investigate dendrimer interactions with small aromatic hydrocarbons using all-atom molecular dynamics simulations. We study the association of naphthalene (NPH)—the smallest polycyclic aromatic hydrocarbon—with 3rd–6th generation (G3–G6) polyamidoamine (PAMAM) dendrimers. Our work emphasizes that the association of small aromatic molecules with PAMAM dendrimers involves the formation of dynamic pocket-like association sites through interactions between flexible dendrimer branches and NPH molecules. The association sites are primarily formed by branches from the two outermost dendrimer subgenerations, and often involve the tertiary amine groups. Irrespective of their location on the dendrimer—whether buried or near the outer surface—these pocket-like structures lower the hydration of the associated NPH molecules. We show that on average NPH molecules with a lower hydration have a greater tendency to remain associated with the dendrimer for longer times. In general, the association sites are similar for the G3–G6 PAMAM dendrimers, indicating similarities in the association mechanisms across different dendrimer generations.

 Please wait while we load your content...
Please wait while we load your content...