Rose Bengal-photosensitized oxidation of 4-thiothymidine in aqueous medium: evidence for the reaction of the nucleoside with singlet state oxygen†
Abstract
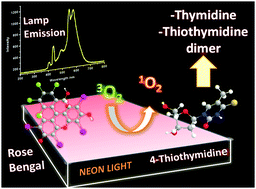
The photoreactivity of 4-thiothymidine (S4TdR) under visible light in the presence of Rose Bengal (RB), acting as a photosensitizer, was investigated in aqueous solutions at pH 7 and 12, using UV-vis, FTIR-ATR and 1H-NMR spectroscopic techniques, time resolved absorption spectroscopy and electrospray ionization mass spectrometry (ESI-MS). Evidence for the generation of thymidine (TdR) as the main product, after one hour of irradiation, was obtained from UV-Vis data, that suggested 4-thiothymidine photodegradation to be faster at basic pH, and confirmed by FTIR-ATR and 1H-NMR data. Clues for the presence of a further product, likely corresponding to a dimeric form of S4TdR, were obtained from the latter techniques. Besides indicating the presence of thymidine, the ESI-MS and MS/MS spectra of the reaction mixtures enabled the identification of the additional product as a S–S bridged covalent dimer of 4-thiothymidine. The concentration of the dimeric species could be estimated with the aid of 1H-NMR data and was found to be lower than that of thymidine in pH 7 reaction mixtures and almost negligible in the pH 12 ones. From a mechanistic point of view, time-resolved absorption spectroscopy measurements provided direct evidence that the formation of the two products cannot be ascribed to a photoinduced electron transfer involving S4TdR and the excited triplet state of RB. Rather, their generation can be interpreted as the result of a bimolecular reaction occurring between singlet state oxygen (1O2), photogenerated by RB, and S4TdR, as demonstrated by the direct detection of 1O2 through IR luminescence spectroscopy. More specifically, a sequential reaction pathway, consisting in the generation of an electrophilic hydroxylated form of S4TdR and its subsequent, rapid reaction with S4TdR, was hypothesized to explain the presence of the S–S bridged covalent dimer of 4-thiothymidine in the reaction mixtures. The described processes make S4TdR an interesting candidate in the role of molecular probe for the detection of 1O2 under different pH conditions.


 Please wait while we load your content...
Please wait while we load your content...