Microwave spectroscopic and theoretical investigations of the strongly hydrogen bonded hexafluoroisopropanol⋯water complex†
Abstract
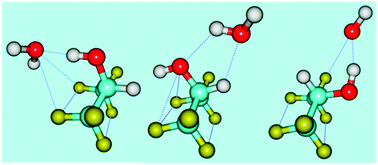
This paper reports microwave spectroscopic and theoretical investigations on the interaction of water with hexafluoroisopropanol (HFIP). The HFIP monomer can exist in two conformations, antiperiplanar (AP) and synclinical (SC). The former is about 5 kJ mol−1 more stable than the latter. Theoretical calculations predicted three potential minima for the complex, two having AP and one having SC conformations. Though, the binding energy for the HFIP(SC)⋯H2O turned out to be larger than that for the other two conformers having HFIP in the AP form, the global minimum for the complex in the potential energy hypersurface had HFIP in the AP form. Experimental rotational constants for four isotopologues measured using a pulsed nozzle Fourier transform microwave spectrometer, correspond to the global minimum in the potential energy hypersurface. The structural parameters and the internal dynamics of the complex could be determined from the rotational spectra of the four isotopologues. The global minimum has the HFIP(AP) as a hydrogen bond donor forming a strong hydrogen bond with H2O. To characterize the strength of the bonding and to probe the other interactions within the complex, atoms in molecules, non-covalent interaction index and natural bond orbital theoretical analyses have been performed.

 Please wait while we load your content...
Please wait while we load your content...