Adsorption of guanidinium collectors on aluminosilicate minerals – a density functional study†
Abstract
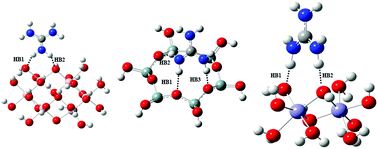
In this density functional theory based investigation, we have modelled and studied the adsorption behaviour of guanidinium cations and substituted (phenyl, methoxy phenyl, nitro phenyl and di-nitro phenyl) guanidinium cationic collectors on the basal surfaces of kaolinite and goethite. The adsorption behaviour is assessed in three different media, such as gas, explicit water and pH medium, to understand the affinity of GC collectors to the SiO4 tetrahedral and AlO6 octahedral surfaces of kaolinite. The tetrahedral siloxane surface possesses a larger binding affinity to GC collectors than the octahedral sites due to the presence of surface exposed oxygen atoms that are active in the intermolecular interactions. Furthermore, the inductive electronic effects of substituted guanidinium cations also play a key role in the adsorption mechanism. Highly positive cations result in a stronger electrostatic interaction and preferential adsorption with the kaolinite surfaces than low positive cations. Computed interaction energies and electron densities at the bond critical points suggest that the adsorption of guanidinium cations on the surfaces of kaolinite and goethite is due to the formation of intra/inter hydrogen bonding networks. Also, the electrostatic interaction favours the high adsorption ability of GC collectors in the pH medium than gas phase and water medium. The structures and energies of GC collectors pave an intuitive view for future experimental studies on mineral flotation.

 Please wait while we load your content...
Please wait while we load your content...