Electrochemical reduction of CO2 to ethylene glycol on imidazolium ion-terminated self-assembly monolayer-modified Au electrodes in an aqueous solution†
Abstract
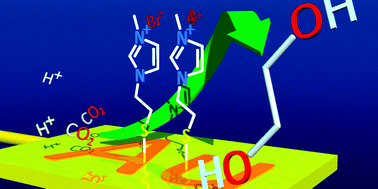
Imidazolium ion-terminated self-assembled monolayer (SAM)-modified electrodes achieve CO2 conversion while suppressing hydrogen evolution. Immobile imidazolium ion on gold (Au) electrodes reduce CO2 at low overpotential. The distance between electrode and imidazolium ion separated by alkane thiol affects CO2 reduction activity. CO2 reduction current depends on the tunnel current rate. Although the product of CO2 reduction at the bare Au electrode is CO, SAM-modified electrodes produce ethylene glycol in aqueous electrolyte solution without CO evolution. The faradaic efficiency reached a maximum of 87%. CO2 reduction at SAM-modified electrodes is unaffected by reduction activity of Au electrode. This phenomenon shows that the reaction field of CO2 reduction is not the electrode surface but the imidazolium ion monolayer.

 Please wait while we load your content...
Please wait while we load your content...