Direct observation of vinyl hydroperoxide†
Abstract
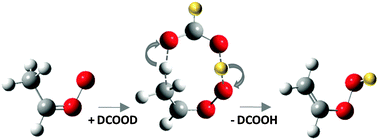
Many alkyl-substituted Criegee intermediates are predicted to undergo an intramolecular 1,4-hydrogen transfer to form isomeric vinyl hydroperoxide species (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) COOH moiety), which break apart to release OH and vinoxy radicals. We report direct detection of stabilized vinyl hydroperoxides formed via carboxylic acid-catalyzed tautomerization of Criegee intermediates. A doubly hydrogen-bonded interaction between the Criegee intermediate and carboxylic acid facilitates efficient hydrogen transfer through a double hydrogen shift. Deuteration of formic or acetic acid permits migration of a D atom to yield partially deuterated vinyl hydroperoxides, which are distinguished from the CH3CHOO, (CH3)2COO, and CH3CH2CHOO Criegee intermediates by mass. Using 10.5 eV photoionization, three prototypical vinyl hydroperoxides, CH2
COOH moiety), which break apart to release OH and vinoxy radicals. We report direct detection of stabilized vinyl hydroperoxides formed via carboxylic acid-catalyzed tautomerization of Criegee intermediates. A doubly hydrogen-bonded interaction between the Criegee intermediate and carboxylic acid facilitates efficient hydrogen transfer through a double hydrogen shift. Deuteration of formic or acetic acid permits migration of a D atom to yield partially deuterated vinyl hydroperoxides, which are distinguished from the CH3CHOO, (CH3)2COO, and CH3CH2CHOO Criegee intermediates by mass. Using 10.5 eV photoionization, three prototypical vinyl hydroperoxides, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOOD, CH2
CHOOD, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)OOD, and CH3CH
C(CH3)OOD, and CH3CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOOD, are detected directly. Complementary electronic structure calculations reveal several reaction pathways, including the barrierless acid-catalyzed tautomerization reaction predicted previously and a barrierless addition reaction that yields hydroperoxy alkyl formate.
CHOOD, are detected directly. Complementary electronic structure calculations reveal several reaction pathways, including the barrierless acid-catalyzed tautomerization reaction predicted previously and a barrierless addition reaction that yields hydroperoxy alkyl formate.

 Please wait while we load your content...
Please wait while we load your content...