Unusual Mn coordination and redox chemistry in the high capacity borate cathode Li7Mn(BO3)3†
Abstract
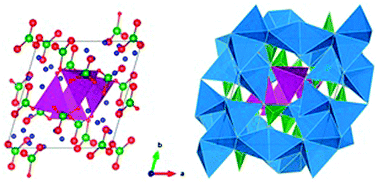
The recently discovered lithium-rich cathode material Li7Mn(BO3)3 has a high theoretical capacity and an unusual tetrahedral Mn2+ coordination. Atomistic simulation and density functional theory (DFT) techniques are employed to provide insights into the defect and redox chemistry, the structural changes upon lithium extraction and the mechanisms of lithium ion diffusion. The most favourable intrinsic defects are Li/Mn anti-site pairs, where Li and Mn ions occupy interchanged positions, and Li Frenkel defects. DFT calculations reproduce the experimental cell voltage and confirm the presence of the unusually high MnV redox state, which corresponds to a theoretical capacity of nearly 288 mA h g−1. The ability to reach the high manganese oxidation state is related to both the initial tetrahedral coordination of Mn and the observed distortion/tilting of the BO3 units to accommodate the contraction of the Mn–O bonds upon oxidation. Molecular dynamics (MD) simulations indicate fast three-dimensional lithium diffusion in line with the good rate performance observed.

 Please wait while we load your content...
Please wait while we load your content...