Ambient preparation and reactions of gas phase silver cluster cations and anions†
Abstract
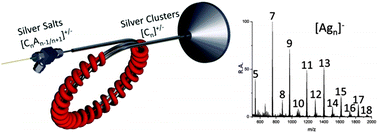
Electrospray ionization of metal salt solutions followed by ambient heating transforms the resulting salt clusters into new species, primarily naked ionic metal clusters. The experiment is done by passing the clusters through a heated coiled loop outside the mass spectrometer which releases the counter-anion while generating the anionic or cationic naked metal cluster. The nature of the anion in the starting salt determines the type of metal cluster observed. For example, silver acetate upon heating generates only positive silver clusters, Agn+, but silver fluoride generates both positive and negative silver clusters, Agn+/− (3 < n < 20). Both unheated and heated metal salt sprays yield ions with characteristic geometric and electronic magic numbers. There is also a strong odd/even effect in the cationic and anionic silver clusters. Thermochemical control is suggested as the basis for favored formation of the observed clusters, with anhydride elimination occurring from the acetates and fluorine elimination from the fluorides to give cationic and anionic clusters, respectively. Data on the intermediates observed as the temperature is ramped support this. The naked metal clusters react with gaseous reagents in the open air, including methyl substituted pyridines, hydrocarbons, common organic solvents, ozone, ethylene, and propylene. Argentation of hydrocarbons, including saturated hydrocarbons, is shown to occur and serves as a useful analytical ionization method. The new cluster formation methodology allows investigation of ligand–metal binding including in reactions of industrial importance, such as olefin epoxidation. These reactions provide insight into the physicochemical properties of silver cluster anions and cations. The potential use of the ion source in ion soft landing is demonstrated by reproducing the mass spectra of salts heated in air using a custom surface science instrument.

 Please wait while we load your content...
Please wait while we load your content...