Fabrication of charged membranes by the solvent-assisted lipid bilayer (SALB) formation method on SiO2 and Al2O3†
Abstract
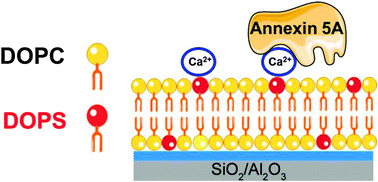
In this study, we employed the solvent-assisted lipid bilayer (SALB) formation method to fabricate charged membranes on solid supports. The SALB formation method exploits a ternary mixture of lipid–alcohol–aqueous buffer to deposit lamellar phase structures on solid supports upon gradual increase of the buffer fraction. Using the quartz crystal microbalance with dissipation (QCM-D) technique, we investigated the formation of negatively and positively charged membranes via the SALB formation method and directly compared with the vesicle fusion method on two different oxide films. Bilayers containing an increasing fraction of negatively charged DOPS lipid molecules were successfully formed on both SiO2 and Al2O3 substrates using the SALB formation method at physiological pH (7.5). In contrast, the vesicle fusion method did not support bilayer formation on Al2O3 and those containing more than 10% DOPS ruptured on SiO2 only under acidic conditions (pH 5). Characterization of the fraction of negatively charge DOPS by in situ annexin 5A binding assay revealed that the fraction of DOPS lipid molecules in the bilayers formed on Al2O3 is significantly higher than that formed on SiO2. This suggests that the SALB self-assembly of charged membranes is predominantly governed by the electrostatic interaction. Furthermore, our findings indicate that when multicomponent lipid mixtures are used, the relative fraction of lipids in the bilayer may differ from the fraction of lipids in the precursor mixture.

 Please wait while we load your content...
Please wait while we load your content...