Geometry controls the stability of FeSi14†
Abstract
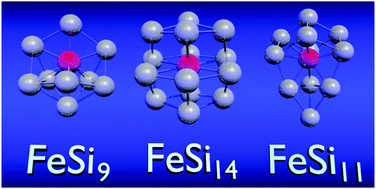
First-principles theoretical studies have been carried out to investigate the stability of Sin cages impregnated with a Fe atom. It is shown that FeSi9, FeSi11, and FeSi14 clusters exhibit enhanced local stability as seen through an increase in Si binding energy, Fe embedding energy, the gap between the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO), and the Ionization Potential (IP). The conventional picture for the stability of such species combines an assumption of electron precise bonding with the 18-electron rule; however, we find this to be inadequate to explain the enhanced stability in FeSi11 and FeSi14 because the d-band is filled for all FeSin clusters for n ≥ 9. FeSi14 is shown to be the most stable due to a compact and highly symmetric Si14 cage with octahedral symmetry that allows better mixing between Fe 3d- and Si 3p-electronic states.

 Please wait while we load your content...
Please wait while we load your content...