Electronically excited states of PANH anions
Abstract
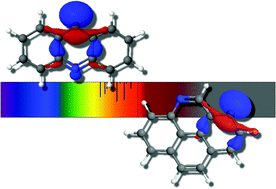
The singly deprotonated anion derivatives of nitrogenated polycyclic aromatic hydrocarbons (PANHs) are investigated for their electronically excited state properties. These include single deprotonation of the two unique arrangements of quinoline producing fourteen different isomers. This same procedure is also undertaken for single deprotonation of the three nitrogenation isomers of acridine and the three of pyrenidine. It is shown quantum chemically that the quinoline-class of PANH anion derivatives can only produce a candidate dipole-bound excited state each, a state defined as the interaction of an extra electron with the dipole moment of the corresponding neutral. However, the acridine- and pyrenidine-classes possess valence excited states as well as the possible dipole-bound excited states where the latter is only possible if the dipole moment is sufficiently large to retain the extra electron; the valence excitation is independent of the radical dipolar strength. As a result, the theoretical vertically computed electronic spectra of deprotonated PANH anion derivatives is fairly rich in the 1.5 eV to 2.5 eV range significantly opening the possibilities for these molecules to be applied to longer wavelength studies of visible and near-IR spectroscopy. Lastly, the study of these systems is also enhanced by the inclusion of informed orbital arrangements in a simply constructed basis set that is shown to be more complete and efficient than standard atom-centered functions.

 Please wait while we load your content...
Please wait while we load your content...