Improving As(iii) adsorption on graphene based surfaces: impact of chemical doping†
Abstract
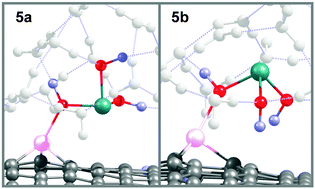
On the basis of quantum chemistry calculations, the adsorption of As(III) onto graphene based adsorbents has been studied. The energetic and molecular properties that characterize the adsorption have been analyzed, and new adsorbents were proposed. The experimentally reported inefficient adsorption of As(III) by intrinsic graphene is theoretically characterized by a low adsorption energy (∼0.3 eV), which is decreased by solvent effects. Two stable conformations were found for the adsorbent–adsorbate systems. The As(III) removal by unmodified oxidized graphene (GO) reaches a medium size adsorption strength (<∼0.8 eV), while still remaining low for high removal efficiency from a water environment. While As(III) adsorption onto boron, nitrogen and phosphorous doped graphene is not favored with respect to the pristine adsorbent, aluminium, silicon and iron embedded graphene can adsorb As(III) by both chemical and physical interactions with high adsorption energies (>∼1 eV), even stable considering a solvent environment. The efficiency of the adsorbents for As(III) removal is sorted as Al–G > Fe–G ≫ Si–G ≫ GO ≫ G. Therefore, Al, Si and Fe doped graphene are considered as potential materials for efficient As(III) removal.

 Please wait while we load your content...
Please wait while we load your content...