Supramolecular amphipathicity for probing antimicrobial propensity of host defence peptides
Abstract
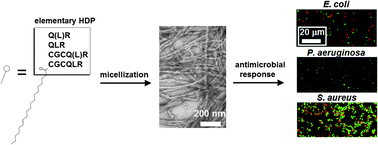
Host defence peptides (HDPs) are effector components of innate immunity that provide defence against pathogens. These are small-to-medium sized proteins which fold into amphipathic conformations toxic to microbial membranes. Here we explore the concept of supramolecular amphipathicity for probing antimicrobial propensity of HDPs using elementary HDP-like amphiphiles. Such amphiphiles are individually inactive, but when ordered into microscopic micellar assemblies, respond to membrane binding according to the orthogonal type of their primary structure. The study demonstrates that inducible supramolecular amphipathicity can discriminate against bacterial growth and colonisation thereby offering a physico-chemical rationale for tuneable targeting of biological membranes.
- This article is part of the themed collection: Chemical compartmentalisation by membranes: from biological mechanism to biomimetic applications

 Please wait while we load your content...
Please wait while we load your content...