Au–Ag nanoalloy molecule-like clusters for enhanced quantum efficiency emission of Er3+ ions in silica
Abstract
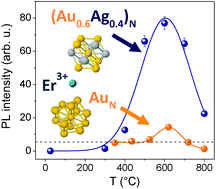
The occurrence of a very efficient non-resonant energy transfer process forming ultrasmall Au–Ag nanoalloy clusters and Er3+ ions is investigated in silica. The enhancement of the room temperature Er3+ emission efficiency by an order of magnitude is achieved by coupling rare-earth ions to molecule-like (AuxAg1−x)N alloy nanoclusters with N = 10–15 atoms and x = 0.6 obtained by optimized sequential ion implantation on Er-implanted silica. For comparison, AuN nanoclusters obtained by the same approach and with the same size and numerical density showed an enhancement by only a factor of 2 with respect to pure Er emission, demonstrating the beneficial effect of using nanoalloyed clusters. The temperature evolution of the energy transfer process is investigated by photoluminescence and exhibits a maximum efficiency at about 600 °C, where the clusters reach the optimal size and the silica matrix completely recovers the implantation damage. The nanoalloy cluster composition and size have been studied by EXAFS analysis, which indicated a stronger Ag–O interaction with respect to the Au–O one and a preferential location of the Ag atoms at the nanoalloy cluster surface.
- This article is part of the themed collection: Recent advances in the chemical physics of nanoalloys

 Please wait while we load your content...
Please wait while we load your content...