Photoinduced molecular dissociation and photoinduced recombination mediated by superfluid helium nanodroplets
Abstract
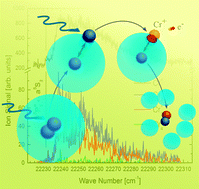
We have investigated photoinduced chemical reaction dynamics of cold, isolated Cr2 molecules in helium nanodroplets (HeN), exploiting the quantum state specific spatial separation of solvated and surface locations on the droplet. The molecules are excited to achieve dissociation to a ground state (a7S3) and a metastable state (a5S2) atom. State specific spatial separation, in combination with efficient translational cooling to avoid ejection, causes the ground state atom to be solvated inside the droplet while the metastable atom migrates to the surface. A barrier between the two reactants formed by the HeN prevents recombination. We apply a resonance-enhanced multiphoton ionization scheme including the 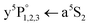 transition of the surface atom as well as a two-laser scheme including the
transition of the surface atom as well as a two-laser scheme including the 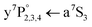 transition of the solvated atom in order to verify the locations and separation of the dissociation products. Furthermore, ionization of the a5S2 surface atom triggers solvation followed by geminate recombination with the a7S3 atom, which is verified by the detection of Cr2+ molecular ions. For small Cr clusters, our results indicate that they may be composed of chromium dimers that exhibit the same dissociation behavior.
transition of the solvated atom in order to verify the locations and separation of the dissociation products. Furthermore, ionization of the a5S2 surface atom triggers solvation followed by geminate recombination with the a7S3 atom, which is verified by the detection of Cr2+ molecular ions. For small Cr clusters, our results indicate that they may be composed of chromium dimers that exhibit the same dissociation behavior.

 Please wait while we load your content...
Please wait while we load your content...