Atomistic modeling to optimize composition and characterize structure of Ni–Zr–Mo metallic glasses
Abstract
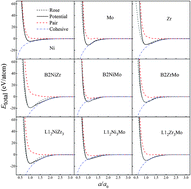
An interatomic potential was constructed for the Ni–Zr–Mo ternary metal system with the newly proposed long-range empirical formulism, which has been verified to be applicable for fcc, hcp and bcc transition metals and their alloys. Applying the constructed potential, molecular dynamics simulations predict a hexagonal composition region within which metallic glass formation is energetically favored. Based on the simulation results, the driving force for amorphous phase formation is derived, and thus an optimized composition is pinpointed to Ni45Zr40Mo15, of which the metallic glass could be most stable or easiest to obtain. Further structural analysis indicates that the dominant interconnected clusters for Ni64Zr36−xMox MGs are 〈0, 0, 12, 0〉, 〈0, 1, 10, 2〉, 〈0, 2, 8, 2〉 and 〈0, 3, 6, 4〉. In addition, it is found that the appropriate addition of Mo content could not only make a more ordered structure with a higher atomic packing density and a lower energy state, but also improve the glass formation ability of Ni–Zr–Mo alloys. Moreover, inherent hierarchical atomic configurations for ternary Ni–Zr–Mo metallic glasses are clarified via the short-range, medium-range and further in the extended scale of the icosahedral network.

 Please wait while we load your content...
Please wait while we load your content...