Nucleation and growth of condensate in nanoporous materials
Abstract
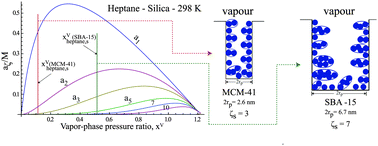
We consider the adsorption–desorption cycles of water and of three hydrocarbons on MCM-41 and on SBA-15. We show that during the desorption portion of a cycle, when the condensate is still at the mouth of the pores, in equilibrium, and the pressure, PVw, is the minimum value reached before pore-emptying begins, the contact angle is zero. This value of the contact angle is used with the Kelvin equation to calculate the pore radius of each of the mesoporous silicas considered. The standard deviations in the values are found to differ by only a few percent. We propose a method for predicting the size of adsorbed-molecular clusters that must be formed in the pores to initiate condensate formation there. Once formed, the condensate grows spontaneously to the pore mouth. If the vapour-phase pressure when this condition is reached is also PVw, the adsorption–desorption cycle is reversible. Three of the eight systems considered meet this condition and their adsorption–desorption cycles are experimentally reversible.

 Please wait while we load your content...
Please wait while we load your content...