Structural characterization of eutectic aqueous NaCl solutions under variable temperature and pressure conditions†
Abstract
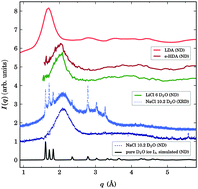
The structure of amorphous NaCl solutions produced by fast quenching is studied as a function of pressure, up to 4 GPa, by combined neutron diffraction experiments and classical molecular dynamics simulations. Similarly to LiCl solutions the system amorphizes at ambient pressure in a dense phase structurally similar to the e-HDA phase in pure water. The measurement of the static structure factor as a function of pressure allowed us to validate a new polarizable force field developed by Tazi et al., 2012, never tested under non-ambient conditions. We infer from simulations that the hydration shells of Na+ cations form well defined octahedra composed of both H2O molecules and Cl− anions at low pressure. These octahedra are gradually broken by the seventh neighbour moving into the shell of first neighbours yielding an irregular geometry. In contrast to LiCl solutions and pure water, the system does not show a polyamorphic transition under pressure. This confirms that the existence of polyamorphism relies on the tetrahedral structure of water molecules, which is broken here.

 Please wait while we load your content...
Please wait while we load your content...