p-Cyanophenylalanine and selenomethionine constitute a useful fluorophore–quencher pair for short distance measurements: application to polyproline peptides†
Abstract
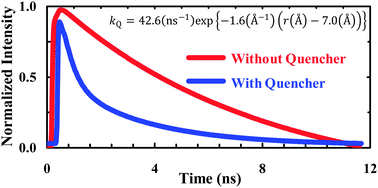
The C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching frequency and fluorescence quantum yield of p-cyanophenylalanine are sensitive to environment. As such, this unnatural amino acid has found broad applications, ranging from studying how proteins fold to determining the local electric field of membranes. Herein, we demonstrate that the fluorescence of p-cyanophenylalanine can be quenched by selenomethionine through an electron transfer process occurring at short distances, thus further expanding its spectroscopic utility. Using this fluorophore–quencher pair, we are able to show that short polyproline peptides (1–4 prolines) are not rigid; instead, they sample a bimodal conformational distribution.
N stretching frequency and fluorescence quantum yield of p-cyanophenylalanine are sensitive to environment. As such, this unnatural amino acid has found broad applications, ranging from studying how proteins fold to determining the local electric field of membranes. Herein, we demonstrate that the fluorescence of p-cyanophenylalanine can be quenched by selenomethionine through an electron transfer process occurring at short distances, thus further expanding its spectroscopic utility. Using this fluorophore–quencher pair, we are able to show that short polyproline peptides (1–4 prolines) are not rigid; instead, they sample a bimodal conformational distribution.

 Please wait while we load your content...
Please wait while we load your content...