Microsolvation of LiBO2 in water: anion photoelectron spectroscopy and ab initio calculations†
Abstract
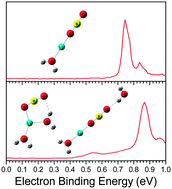
The microsolvation of LiBO2 in water was investigated by conducting anion photoelectron spectroscopy and ab initio studies on the LiBO2(H2O)n− (n = 0–5) clusters. By comparing calculations with experiments, the structures of these clusters and their corresponding neutrals were assigned, and their structural evolutions were revealed. During the anionic structural evolution with n increasing to 5, hydroxyborate and metaborate channels were identified and the metaborate channel is more favorable. For the hydroxyborate structures, the anionic Li+–BO2− ion pair reacts with a water molecule to produce the LiBO(OH)2− moiety and three water molecules tend to dissolve this moiety. In the metaborate channel, two types of solvent-separated ion pair (SSIP) geometries were determined as the ring-type and linear-type. The transition from the contact ion pair (CIP) to the ring-type of SSIP starts at n = 3, while that to the linear-type of SSIP occurs at n = 4. In neutral LiBO2(H2O)n clusters, the first water molecule prefers to react with the Li+–BO2− ion pair to generate the LiBO(OH)2 moiety, analogous to the bulk crystal phase of α-LiBO2 with two O atoms substituted by two OH groups. The Li–O distance in the LiBO(OH)2 moiety increases with the increasing number of water molecules and elongates abruptly at n = 4. Our studies provide new insight into the initial dissolution of LiBO2 salt in water at the molecular level and may be correlated to the bulk state.

 Please wait while we load your content...
Please wait while we load your content...