Pore collapse and regrowth in silicon electrodes for rechargeable batteries†
Abstract
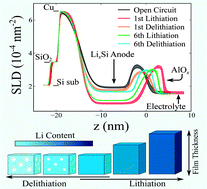
Structure and composition of an 11 nm thick amorphous silicon (a-Si) thin film anode, capped with 4 nm of alumina are measured, in operando, by neutron reflectivity (NR) and electrochemical impedance spectroscopy in a lithium half-cell. NR data are analyzed to quantify the a-Si thickness and composition at various states of charge over six cycles. The a-Si anode expands and contracts upon lithiation and delithiation, respectively, while maintaining its integrity and low interfacial roughness (≤1.6 nm) throughout the cycling. The apparently non-linear expansion of the a-Si layer volume versus lithium content agrees with previous thin-film a-Si anode studies. However, a proposed pore collapse and regrowth (PCRG) mechanism establishes that the solid domains in the porous LixSi film expand linearly with Li content at 8.48 cm3 mol−1 Li, similar to crystalline Si. In the PCRG model, porosity is first consumed by expansion of solid domains upon lithiation, after which the film as a whole expands. Porosity is reestablished at 5–28% upon delithiation. Data show that the alumina protective layer on the a-Si film functions as an effective artificial solid electrolyte interphase (SEI), maintaining its structural integrity, low interfacial roughness, and relatively small transport resistance. No additional spontaneously-formed SEI is observed in this study.

 Please wait while we load your content...
Please wait while we load your content...