Biophysics of α-synuclein induced membrane remodelling†
Abstract
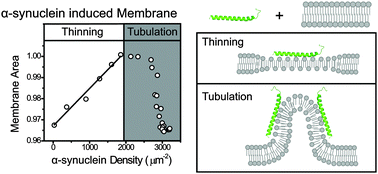
α-Synuclein is an intrinsically disordered protein whose aggregation is a hallmark of Parkinson's disease. In neurons, α-synuclein is thought to play important roles in mediating both endo- and exocytosis of synaptic vesicles through interactions with either the lipid bilayer or other proteins. Upon membrane binding, the N-terminus of α-synuclein forms a helical structure and inserts into the hydrophobic region of the outer membrane leaflet. However, membrane structural changes induced by α-synuclein are still largely unclear. Here we report a substantial membrane area expansion induced by the binding of α-synuclein monomers. This measurement is accomplished by observing the increase of membrane area during the binding of α-synuclein to pipette-aspirated giant vesicles. The extent of membrane area expansion correlates linearly with the density of α-synuclein on the membrane, revealing a constant area increase induced by the binding per α-synuclein molecule. The area expansion per synuclein is found to exhibit a strong dependence on lipid composition, but is independent of membrane tension and vesicle size. Fragmentation or tubulation of the membrane follows the membrane expansion process. However, contrary to BAR domain proteins, no distinct tubulation-transition density can apparently be identified for α-synuclein, suggesting a more complex membrane curvature generation mechanism. Consideration of α-synuclein's membrane binding free energy and biophysical properties of the lipid bilayer leads us to conclude that membrane expansion by α-synuclein results in thinning of the bilayer. These membrane thinning and tubulation effects may underlie α-synuclein's role in mediating cell trafficking processes such as endo- and exocytosis.
- This article is part of the themed collection: Chemical compartmentalisation by membranes: from biological mechanism to biomimetic applications

 Please wait while we load your content...
Please wait while we load your content...