Native silica nanoparticles are powerful membrane disruptors
Abstract
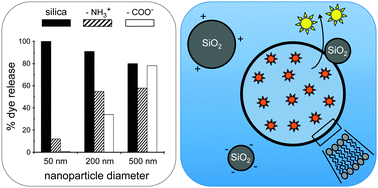
Silica nanoparticles are under development for intracellular drug delivery applications but can also have cytotoxic effects including cell membrane damage. In this study, we investigated the interactions of silica nanospheres of different size, surface chemistry and biocoating with membranes of phosphatidylcholine lipids. In liposome leakage assays many, but not all, of these nanoparticles induced dose-dependent dye leakage, indicative of membrane perturbation. It was found that 200 and 500 nm native-silica, aminated and carboxylated nanospheres induce near-total dye release from zwitterionic phosphatidylcholine liposomes at a particle/liposome ratio of ∼1, regardless of their surface chemistry, which we interpret as particle-supported bilayer formation following a global rearrangement of the vesicular membrane. In contrast, 50 nm diameter native-silica nanospheres did not induce total dye leakage below a particle/liposome ratio of ∼8, whereas amination or carboxylation, respectively, strongly reduced or prevented dye release. We postulate that for the smaller nanospheres, strong silica–bilayer interactions are manifested as bilayer engulfment of membrane-adsorbed particles, with localized lipid depletion eventually leading to collapse of the vesicular membrane. Protein coating of the particles considerably reduced dye leakage and lipid bilayer coating prevented dye release all together, while the inclusion of 33% anionic lipids in the liposomes reduced dye leakage for both native-silica and aminated surfaces. These results, which are compared with the effect of polystyrene nanoparticles and other engineered nanomaterials on lipid bilayers, and which are discussed in relation to nanosilica-induced cell membrane damage and cytotoxicity, indicate that a native-silica nanoparticle surface chemistry is a particularly strong membrane interaction motif.
- This article is part of the themed collection: Chemical compartmentalisation by membranes: from biological mechanism to biomimetic applications

 Please wait while we load your content...
Please wait while we load your content...